Nesosilicates (Island Silicates)
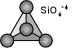 Oxygens atoms are not shared with other SiO4-4
tetrahedrons. Ratio between Si & O is 1:4. The basic structural unit
is SiO4-4. Eg.
Olivine and Garnet group
(Mg,Fe)2SiO4.
Oxygens atoms are not shared with other SiO4-4
tetrahedrons. Ratio between Si & O is 1:4. The basic structural unit
is SiO4-4. Eg.
Olivine and Garnet group
(Mg,Fe)2SiO4.
minerallogy
The atomic structure of nesosilicates are generally dense
which cause the mineral of this group to have high specific gravity and hardness. Crystal system is generally equidimensional and have poor cleavage.
Sorosilicates ( Double Island Silicatesy )
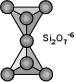 One oxygens is shared with another tetrahedron.Si:O is 2:7,The basic structural unit
is Si2O7-6. Eg. Epidote group (Si2O7)
One oxygens is shared with another tetrahedron.Si:O is 2:7,The basic structural unit
is Si2O7-6. Eg. Epidote group (Si2O7)
Cyclosilicates (Ring Silicates)
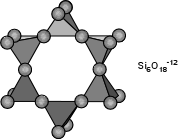
Two oxygens are shared and the structure is arranged in a ring, such as that shown here, Si:O is 1:3. Si6O18-12. Eg. Beryl - Be3Al2Si6O18.
 Inosilicates (Single Chain Silicates)
Inosilicates (Single Chain Silicates)
If two of the oxygens are shared in a way to make long single chains of linked SiO4 tetrahedra, we get the single chain silicates or inosilicates. In this case the basic structural unit is Si2O6-4 or SiO3-2. Eg. pyroxene group, like the orthopyroxenes (Mg,Fe)SiO3 or the clinopyroxenes Ca(Mg,Fe)Si2O6.
Inosilicates (Double Chain Silicates )
If two chains are linked together so that each tetrahedral group shares 3 of its oxygens, we can from double chains, with the basic structural group being Si4O11-6. Eg. amphibole group like tremolite - ferroactinolite series - Ca2(Mg,Fe)5Si8O22(OH)2.
Phyllosilicates (Sheet Silicates)
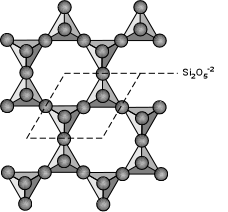
3 oxygens are shared, basic structural group is Si2O5-2. Eg. micas, clay minerals, chlorite, talc, and serpentine minerals. A good example is biotite - K(Mg,Fe)3(AlSi3)O10(OH)2. Note that in this structure, Al is substituting for Si in one of the tetrahedral groups.
Tectosilicates (Framework Silicates)
 All oxygens are shared with another SiO4
tetrahedron, The basic
structural SiO2. The minerals quartz,
cristobalite, and tridymite all are based on this structure. If some
of the Si+4 ions are replaced by Al+3 then this
produces a charge imbalance and allows for other ions to be found
coordinated in different arrangements within the framework
structure. Thus, the feldspar and feldspathoid minerals are also
based on the tectosilicate framework.
All oxygens are shared with another SiO4
tetrahedron, The basic
structural SiO2. The minerals quartz,
cristobalite, and tridymite all are based on this structure. If some
of the Si+4 ions are replaced by Al+3 then this
produces a charge imbalance and allows for other ions to be found
coordinated in different arrangements within the framework
structure. Thus, the feldspar and feldspathoid minerals are also
based on the tectosilicate framework.
Sorosilicates ( Double Island Silicatesy )
 One oxygens is shared with another tetrahedron.Si:O is 2:7,The basic structural unit
is Si2O7-6. Eg. Epidote group (Si2O7)
One oxygens is shared with another tetrahedron.Si:O is 2:7,The basic structural unit
is Si2O7-6. Eg. Epidote group (Si2O7)Cyclosilicates (Ring Silicates)

Two oxygens are shared and the structure is arranged in a ring, such as that shown here, Si:O is 1:3. Si6O18-12. Eg. Beryl - Be3Al2Si6O18.
 Inosilicates (Single Chain Silicates)
Inosilicates (Single Chain Silicates)If two of the oxygens are shared in a way to make long single chains of linked SiO4 tetrahedra, we get the single chain silicates or inosilicates. In this case the basic structural unit is Si2O6-4 or SiO3-2. Eg. pyroxene group, like the orthopyroxenes (Mg,Fe)SiO3 or the clinopyroxenes Ca(Mg,Fe)Si2O6.
Inosilicates (Double Chain Silicates )
If two chains are linked together so that each tetrahedral group shares 3 of its oxygens, we can from double chains, with the basic structural group being Si4O11-6. Eg. amphibole group like tremolite - ferroactinolite series - Ca2(Mg,Fe)5Si8O22(OH)2.
Phyllosilicates (Sheet Silicates)

3 oxygens are shared, basic structural group is Si2O5-2. Eg. micas, clay minerals, chlorite, talc, and serpentine minerals. A good example is biotite - K(Mg,Fe)3(AlSi3)O10(OH)2. Note that in this structure, Al is substituting for Si in one of the tetrahedral groups.
Tectosilicates (Framework Silicates)
 All oxygens are shared with another SiO4
tetrahedron, The basic
structural SiO2. The minerals quartz,
cristobalite, and tridymite all are based on this structure. If some
of the Si+4 ions are replaced by Al+3 then this
produces a charge imbalance and allows for other ions to be found
coordinated in different arrangements within the framework
structure. Thus, the feldspar and feldspathoid minerals are also
based on the tectosilicate framework.
All oxygens are shared with another SiO4
tetrahedron, The basic
structural SiO2. The minerals quartz,
cristobalite, and tridymite all are based on this structure. If some
of the Si+4 ions are replaced by Al+3 then this
produces a charge imbalance and allows for other ions to be found
coordinated in different arrangements within the framework
structure. Thus, the feldspar and feldspathoid minerals are also
based on the tectosilicate framework.
No comments:
Post a Comment